Water, the substance that covers about 71% of Earth’s surface and constitutes around 60% of the human body, is essential to life as we know it. Despite its ubiquity, water’s unique properties often go unnoticed in our daily lives. From quenching our thirst to regulating our planet’s climate, water’s remarkable characteristics play critical roles in both natural processes and human activities. This article delves into the fascinating properties of water, exploring how these properties manifest in its three states—ice, liquid, and steam—and why they are so vital to our world.
I. The Molecular Structure of Water
A. Water Molecule Composition
Water’s chemical formula, H2O, reveals that each molecule comprises two hydrogen atoms bonded to one oxygen atom. The covalent bonds that hold these atoms together are responsible for water’s unique properties. In a water molecule, the hydrogen atoms form an angle of approximately 104.5 degrees with the oxygen atom, creating a bent shape. This molecular structure is crucial to understanding water’s behavior.
B. Polarity of Water Molecules
The oxygen atom in a water molecule is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This difference in electronegativity creates a polar molecule with a partial negative charge near the oxygen atom and partial positive charges near the hydrogen atoms. The polarity of water molecules allows them to form hydrogen bonds with each other, contributing to many of water’s unique physical and chemical properties.
II. Physical Properties of Water
A. States of Matter
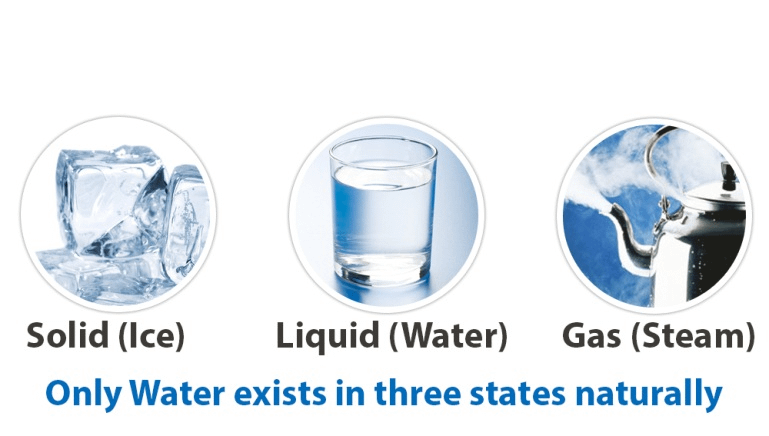
Water exists in three states: solid (ice), liquid (water), and gas (steam). Each state has distinct characteristics influenced by temperature and pressure. As a solid, water forms a crystalline structure that is less dense than its liquid form, which is why ice floats. As a liquid, water is a versatile solvent and medium for various biological and chemical processes. In its gaseous state, water vapor plays a crucial role in weather patterns and the water cycle.
B. Density and Expansion
One of the most intriguing properties of water is its density behavior. Unlike most substances, water is densest at 4°C. As it cools below this temperature, it begins to expand, resulting in ice that is less dense than liquid water. This expansion upon freezing is essential for aquatic life, as it ensures that ice forms on the surface of bodies of water, insulating the liquid below and allowing life to survive in cold conditions.
C. High Specific Heat Capacity
Water has a high specific heat capacity, meaning it can absorb or release a significant amount of heat with only a slight change in its temperature. This property is vital for maintaining stable temperatures in natural environments and living organisms. For instance, large bodies of water like oceans and lakes can moderate climate by absorbing heat during the day and releasing it at night, thus preventing extreme temperature fluctuations.
D. High Heat of Vaporization
The high heat of vaporization of water means that it requires a lot of energy to change from a liquid to a gas. This property is critical for cooling mechanisms in living organisms. When we sweat, the evaporation of water from our skin removes excess heat from our bodies, helping to regulate our temperature. Similarly, plants use transpiration to cool themselves and maintain water balance.
III. Chemical Properties of Water
A. Solvent Capabilities
Water is often called the “universal solvent” because it can dissolve a wide range of substances. This capability is due to the polarity of water molecules, which can surround and interact with various ions and polar molecules. Water’s solvent properties are crucial for many biological processes, including nutrient transport, waste removal, and chemical reactions within cells.
B. pH and Dissociation
Water plays a significant role in maintaining pH balance in various environments. Pure water has a neutral pH of 7, but it can dissociate into hydrogen ions (H+) and hydroxide ions (OH-), which are essential for many chemical reactions. The self-ionization of water, although minimal, is vital for the acid-base balance in biological systems and industrial processes.
C. Reactivity
Water is involved in many chemical reactions, such as hydrolysis, where it breaks down complex molecules into simpler ones. This reactivity is essential for digestion and various metabolic processes. Additionally, water’s ability to react with different substances, including acids, bases, and salts, makes it a critical component in numerous chemical and industrial applications.
IV. Biological Significance of Water Properties
A. Cellular Functions
Water is fundamental to the structure and function of cells. It constitutes the majority of cell mass and provides a medium for biochemical reactions. Water’s ability to dissolve various substances allows for the efficient transport of nutrients and waste products within cells and across cell membranes.
B. Temperature Regulation
Water’s high specific heat capacity and heat of vaporization are essential for regulating body temperature in living organisms. These properties allow organisms to maintain homeostasis by dissipating excess heat through processes like sweating and respiration. For example, humans sweat to cool down, and dogs pant to regulate their body temperature.
C. Transport Medium
Water serves as a crucial transport medium in both animals and plants. In animals, water is a major component of blood, which transports oxygen, nutrients, and waste products throughout the body. In plants, water is essential for the movement of nutrients and minerals from the soil to various parts of the plant through the xylem and phloem.
V. Ecological Impact of Water Properties
A. Water Cycle
The water cycle, or hydrological cycle, is a continuous process that involves the movement of water on, above, and below the Earth’s surface. Key processes include evaporation, condensation, precipitation, and collection. The water cycle is vital for replenishing freshwater sources, supporting plant and animal life, and regulating climate patterns.
B. Aquatic Life
Water’s unique properties are essential for the survival of aquatic life. The ability of ice to float provides insulation for aquatic organisms during winter, while water’s solvent properties ensure that essential nutrients and gases are available for life processes. Additionally, the high specific heat capacity of water helps maintain stable temperatures in aquatic environments, crucial for the survival of marine and freshwater species.
C. Ice and Climate
Ice plays a significant role in regulating global temperatures. Polar ice caps and glaciers reflect sunlight, helping to cool the Earth. However, melting ice due to climate change leads to rising sea levels and altered weather patterns, posing significant challenges for ecosystems and human populations.
VI. Human Utilization and Challenges
A. Water in Daily Life
Water is indispensable in our daily lives. We use it for drinking, cooking, cleaning, and sanitation. Additionally, water is critical in agriculture for irrigation and livestock, ensuring food security for the global population.
B. Technological Applications
Water is essential in various technological and industrial applications. It is used in power generation, including hydroelectricity and steam turbines. Water is also crucial in manufacturing processes, chemical production, and as a coolant in nuclear reactors.
C. Water Scarcity and Pollution
Despite its abundance, clean, fresh water is a finite resource. Many regions face water scarcity due to overuse, pollution, and climate change. Contaminants like chemicals, plastics, and waste pose significant threats to water quality. Addressing these challenges requires sustainable water management practices, pollution control measures, and conservation efforts to ensure that future generations have access to this vital resource.
Conclusion
Water, from its molecular structure to its multifaceted properties, is truly a marvel of nature. Its ability to exist in three states—ice, liquid, and steam—and its unique physical and chemical characteristics make it indispensable for life, ecosystems, and human civilization. As we continue to explore and understand water’s properties, it becomes increasingly clear that conserving and managing this precious resource is crucial for our planet’s health and our own survival.
References
- National Oceanic and Atmospheric Administration (NOAA). (n.d.). The Water Cycle. Retrieved from NOAA
- United States Geological Survey (USGS). (n.d.). Water Properties Information. Retrieved from USGS
- NASA Earth Observatory. (n.d.). Water Cycle. Retrieved from NASA
- National Aeronautics and Space Administration (NASA). (n.d.). The Properties of Water. Retrieved from NASA
- Environmental Protection Agency (EPA). (n.d.). Water Quality. Retrieved from EPA





